Recombinant Human Interleukin-4
CERTIFICATE OF ANALYSIS
| Source | E.coli |
| Appearance | Colourless, clear liquid free of particles |
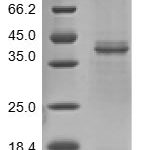
| Identity | 1 band at 15kDa as measured by SDS-PAGE/Western Blot |
| Specific activity | 12.4 x 10 exp6 units / mg compared to NIBSC standard (Bioassay) |
| Endotoxin content | < 0.1EU/µg (LAL) |
| Protein content | 50±10 µg/vial (Lowry/µBCA) |
| Trehalose | 6±0.5 mg/ml (HPLC) |
| Sterility | Absence of growth (FTM (30-35°C)) / Absence of growth (TSB (20-25°C)) |
| Abnormal toxicity | No weight loss, no abnormal reaction in mice |
| General Growth | No weight loss, no abnormal reaction in guinea pigs |
| Physical state | Freeze-dried |
| Stability | 12 months at -20°C to -80°C At least 3 months after reconstruction when stored at -20°C to -80°C |
| Reconstruction | Use 500µL water for injection |
| Packaging unit | 50 µg protein (Lowry test) |
| Purity | >98% as determined by SDS-PAGE and HPLC |
GMP certification: GENTAUR rh IL-4 is manufactured in full compliance with cGMP in facilities approved by the Belgian Ministry of Health for the production and storage of medicinal products. The manufacturing process does not involve the use of products of animal origin.
BSE/TSE Declaration: GENTAUR BVBA declares that this recombinant human IL-4 (04-GMPhuIL4) is manufactured under strict GMP controls and certifies that the entire product line is BSE (Bovine Spongiform Encephalopathy) and TSE (Transmissible Spongiform Encephalopathy) free. GENTAUR BVBA manufactures this product in Belgium.
Use GENTAUR rh IL-4 is not an approved medicinal product and ONLY be injected as such to patients after the ethical commission approval of your university.
More information:
Recombinant Human InterleukiF.pdf
IL4 detail.pdf
rHuIL-4.pdf
For More Information Contact us